Latest videos
急性坏疽阑尾炎的手术治疗
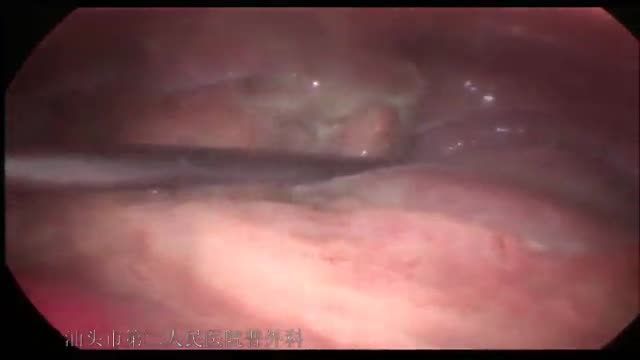
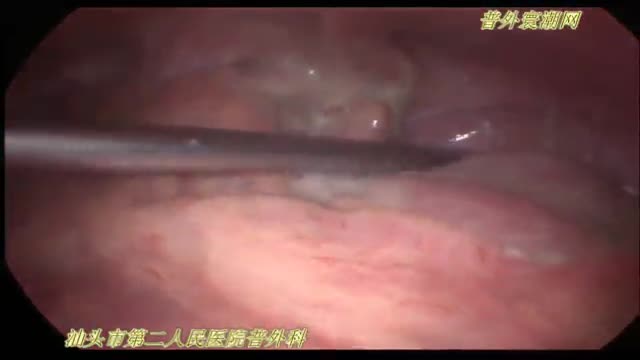
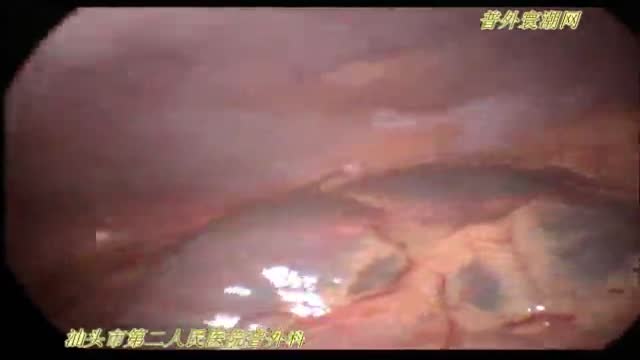
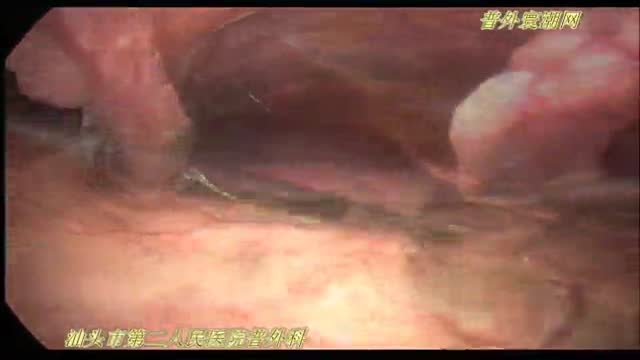
急性坏疽性阑尾炎并穿孔de 腹腔镜手术治疗
Laparoscopic duodenal ulcer perforation repair 2
腹腔镜十二指肠球部溃疡穿孔修补术
Coconut Oil Provides Thyroid Gland Benefits
Anti Aging Diet
Asthma Mechanism 3D
Digestive System Animation
Diabetes Insipidus Symptoms
Bone Movement During Childbirth and Delivery 3D
The Role of Insulin in the Human Body
Diabetes Animation 3D
Type 2 Diabetes Causes Symptoms and Treatment
How to Reverse Type 2 Diabetes
How to Know If You Have Diabetes
Type 2 Diabetes Animation 3D
Insulin Processes Mechanism Animation 3D
Stroke Animation 3D
Erectile Dysfunction Information 3D Animation
Brain Anatomy and Functions Animation
Showing 272 out of 273