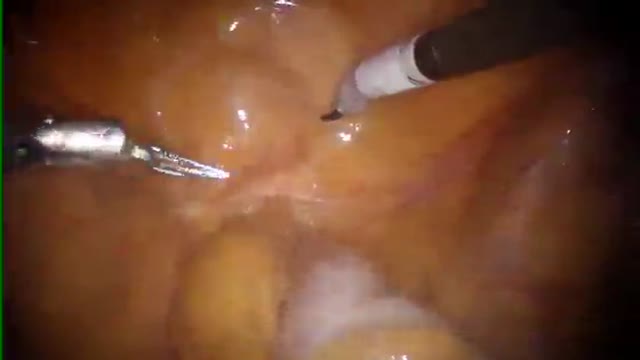
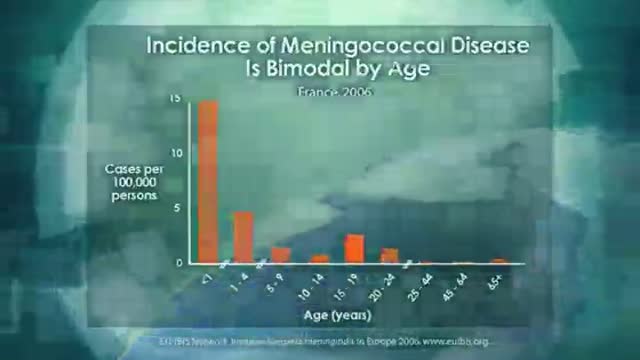
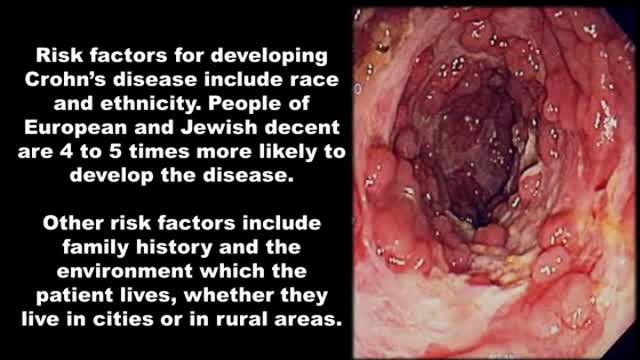
New developed antibody
0
0
1,868 ভিউ·
01/17/24
A new report analyzing FDA-approved monoclonal antibodies (mAbs) produced by a select group of leading biotechnology companies shows that clinical development times – specifically the duration of Phase II and Phase III trials – are lengthening, while FDA review times have remained constant. The average time from investigational new drug (IND) filing to market was 6.7 years for 11 mABs approved between 1994 and 2003 but shot up to 8.3 years for 12 mAbs approved between 2004 and March 9, 2011, according to Deloitte Recap LLC’s analysis, Therapeutic Monoclonal Antibodies – Insights, Strategies and Data.
আরো দেখুন
0 মন্তব্য
sort ক্রমানুসার